A close collaboration of scientific writers and biostatisticians to design your research project and communicate…

Risk-based monitoring
An agile approach to improve data quality, ensure study continuity and reduce site burden.
We develop monitoring plans that address the clinical project specificities with the best cost-efficiency.
RISK-BASED MONITORING:
RISK ANALYSIS AND ASSESSMENT
TO DEFINE THE BEST MONITORING STRATEGY
Depending on the nature of the clinical project (interventional or not), its complexity and the type of investigators involved, we conduct a risk analysis and assessment for all studies in order to define the best risk-based monitoring strategy.
Based on this analysis, we draw up a realistic risk-based monitoring plan and adjust the number and type of monitoring visits (on-site, remote or decentralised), their frequency, their triggering and the percentage of source data verification (SDV).

On-site Monitoring
On site-monitoring is an in-person evaluation carried out by the ICTA monitor at an investigational site where the clinical investigation is being conducted.

Remote Monitoring
Remote monitoring is an off-site evaluation performed by the ICTA monitor away from the site where the clinical investigation is being conducted.

Centralised Monitoring
Centralised monitoring is a site evaluation and source data verification carried out by the ICTA monitor at a central location other than the site where the clinical investigation is being conducted. This requires the use of secure digital tools to support communication between stakeholders and the sharing of sensitive documents using an electronic safe.
RISK-BASED MONITORING APPROACH
Risk Management is part of our philosophy. Identifying the major risks (Key Risk Indicators) and critical data is key to building a comprehensive, centralised and dynamic risk-based monitoring plan.
The components of Risk-Based Monitoring are broader than just focusing on the balance between on-site and remote activities.
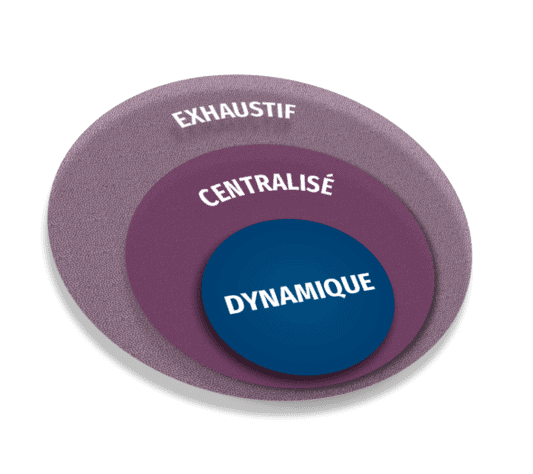
COMPREHENSIVE
Package of measures among which on-site monitoring is only a portion
It encompasses data review and statistical analysis
CENTRALISED
Comparison of data at global results level and individual sites level
It emphasizes on remote data review and site management contacts
DYNAMIC
The monitoring planning process is iterative, ongoing, and based on the project needs determined by the analysis of objective and subjective indicators
3 categories of risk
Indicators are defined during the risk assessment and planning phase, in comparison with the standard of care and risk of GCP/GPE non-compliance. 3 categories of risk should be considered:
- PROTOCOL
- DATA
- SITE
Protocol and data-level risks determine the intensity, frequency, types, and timing of monitoring activities, whereas site-level risks account for timing, tasks and type of monitoring activities.
With this in mind, we adapt our monitoring guidelines to each project, in accordance with the critical parameters of the study, the early-warning signals, the specificities of safety monitoring. We also train our Clinical Research Assistants (CRAs), for each new study, to on-site/remote monitoring activities, to follow the corrective actions put in place, and, in a general way, to anticipate problems by providing sites with the necessary support. This last aspect is facilitated by the geographical location of our monitors, distributed throughout Europe, mainly on the outskirts of major cities.
In order to better manage potential risks, our project managers assess the needs of the investigation sites on a monthly basis and organise, or even modulate if necessary, the CRA visits to focus on the most sensitive sites, according to predefined factors. This assessment takes into account all the information shared in real time on ICTA CTMS via its i-SIT platform, and allows the project team to monitor in real-time investigation sites and patient data, and thus to cross-reference quality markers between monitoring activities and data management.
The best strategy for risk management primarily depends on the type of project, and results from a combination of the methodologies described above.
The different approaches of clinical trial monitoring can be used together for effective oversight and monitoring and for the protection of data integrity and patient safety.
Other services